Trial Findings
The primary results of the AIRWAYS-2 trial have now been published.
Full details are available in the Journal of the American Medical Association (JAMA): Effect of a Strategy of a Supraglottic Airway Device vs Tracheal Intubation During Out-of-Hospital Cardiac Arrest on Functional Outcome, The AIRWAYS-2 Randomized Clinical Trial.
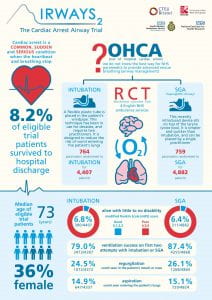
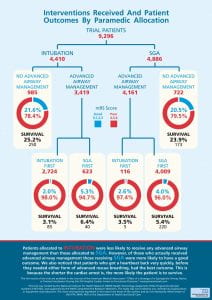
The trial found no significant difference between tracheal intubation and the i-gel supraglottic airway device (SGA) in functional outcome at hospital discharge or 30 days after an out of hospital cardiac arrest in adults. However, some differences were noted in the secondary and pre-specified sensitivity analyses. A trial infographic can be seen below:
An animated video has been produced with www.sciencesplained.com, which can be seen below:
You can see videos from the Paramedic Dissemination Event held in London, by going to the South Western Ambulance Service NHS Foundation Trust YouTube channel.
The long-term outcomes of the trial have been published and are available in Resuscitation. The trial found no significant difference between tracheal intubation and the i-gel supraglottic airway device groups at three and six months after cardiac arrest. This supports the initially reported finding of no difference at 30 days post cardiac arrest/hospital discharge.
What is the AIRWAYS-2 trial?
AIRWAYS-2 is a randomised trial which is comparing the clinical and cost effectiveness of the i-gel supraglottic airway device with tracheal intubation in the initial airway management of patients who have suffered an out of hospital cardiac arrest (OHCA).
Why is this trial being done, and why is it important to the NHS?
A cardiac arrest occurs when the heartbeat and breathing stop suddenly. It is one of the most extreme medical emergencies. Health outcomes are poor; less than 1 in 10 patients survive to be discharged from hospital. The best initial treatment is cardiopulmonary resuscitation (CPR); a combination of rescue breathing and chest compressions. Prompt and effective CPR prevents damage to the brain and other organs, and maximises the chance that the heart will start beating again. Ensuring a clear airway, whilst interrupting chest compressions as little as possible, is essential for survival.
At the moment, we do not know the best way for NHS ambulance staff to provide rescue breathing during an out of hospital cardiac arrest. Placing a breathing tube in the windpipe (tracheal intubation) has been considered the best method. However, attempting to place the breathing tube can cause complications as well as interruptions in chest compressions.
National recommendations suggest using a newer method of airway management: the insertion of a supraglottic airway device. This is a tube that sits on top of the voice box. Supraglottic airway devices are already used during routine anaesthesia in hospital; in emergency care, they are quicker to insert and cause less interruption to chest compressions. However, a supraglottic airway device may not stay in place as securely as a breathing tube and, if a patient vomits, stomach contents may get into their lungs. There is real uncertainty amongst paramedics and experts in the field about the best method to ensure a clear airway during the early stages of OHCA.
We are therefore undertaking a large research study to determine whether intubation or the best available supraglottic airway device (called the i-gel) gives the best chance of recovery following OHCA. It is hoped that the results from this study will shape future OHCA guidelines and will yield real benefits to future OHCA patients in the UK and throughout the world.
Who can get involved in the Airways-2 trial?
The study is being carried out in four English NHS ambulance services:
– South Western Ambulance Service NHS Foundation Trust
– East of England Ambulance Service NHS Trust
– East Midlands Ambulance Service NHS Trust
– Yorkshire Ambulance Service NHS Trust
The study is open to paramedics employed within these trusts who are involved in general operational duties, and could therefore be despatched to attend an OHCA as the first or second paramedic to arrive on scene.
Patients who have a cardiac arrest outside hospital AND who are treated by one of the paramedics who are taking part in AIRWAYS-2 are automatically enrolled in the study due to the urgent need for treatment.
If you are a patient or relative of a patient enrolled in AIRWAYS-2 please click here for further information for patients
Funding: This project was funded by the National Institute for Health Research [Health Technology Assessment Programme] (project number 12/167/102) The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA, NIHR, NHS or the Department of Health. The trial protocol is published on the NIHR website.
Trial Registration: ISRCTN 08256118 – full details can be found on the ISRCTN website.
Ethics Committee Approval: This study has been given a favourable opinion by the South Central-Oxford C Research Ethics Committee: REC number: 14/SC/1219